A
refinery is a production facility composed of a group of
chemical engineering unit processes and unit operations used for refining certain materials or converting raw material into products of value.
Types of refineries :
The various types of refineries include:
*
Oil refinery: Converts petroleum crude oil into high-octane motor fuel (gasoline/petrol), diesel oil, liquefied petroleum gases (LPG), jet aircraft fuel, kerosene, heating fuel oils, lubricating oils, asphalt and petroleum coke.
*
Sugar refinery: Converts sugar cane and sugar beets into crystallized sugar and sugar syrups.
*
Natural gas processing plant: Purifies and converts raw natural gas into residential, commercial and industrial fuel gas, and also recovers natural gas liquids (NGL) such as ethane, propane, butanes and pentanes.
*
Salt refinery: Cleans salt (NaCl), produced by the solar evaporation of sea water, followed by washing and re-crystallization.
*
Various metal refineries such as alumina, copper, gold, lead, nickel, silver, uranium, and zinc.
*
Vegetable oil refineryA typical oil refinery :The image below is a schematic flow diagram of a typical oil refinery that depicts the various unit processes and the flow of intermediate product streams that occurs between the inlet crude oil feedstock and the final end products. The diagram depicts only one of the literally hundreds of different oil refinery configurations. It does not include any of the usual refinery facilities providing utilities such as steam, cooling water, and electric power as well as storage tanks for crude oil feedstock and for intermediate products and end products.
A typical natural gas processing plant :The image below is a schematic block flow diagram of a typical natural gas processing plant. It shows the various unit processes used to convert raw natural gas into sales gas pipelined to the end user markets.
The block flow diagram also shows how processing of the raw natural gas yields byproduct sulfur, byproduct ethane, and natural gas liquids (NGL) propane, butanes and natural gasoline (denoted as pentanes +).
Typical refining of sugar :
Most of the sugar produced worldwide is derived either from sugarcane or sugar beets. However, the sugar produced from sugarcane is at least twice the amount produced by sugar beets. For that reason, this section on the refining of sugar deals with sugar produced from sugarcane..
Milling The refining of sugarcane into sugar has traditionally been done in two stages. The first stage is the production of a raw sugar by the milling of freshly harvested sugarcane, usually done locally in the sugarcane-producing regions. In a sugar mill, sugarcane is washed, chopped, and shredded by revolving knives. The shredded cane is mixed with water and crushed. The juices (containing 10-15 percent sucrose) are collected and mixed with lime to adjust its pH to 7 which arrests sucrose's decay into glucose and fructose, and precipitates out some impurities. The lime and other suspended solids are settled out, and the clarified juice is concentrated in a multiple-effect evaporator to make a syrup with about 60 weight percent sucrose. The syrup is further concentrated under vacuum until it becomes supersaturated, and then seeded with crystalline sugar. Upon cooling, sugar crystallizes out of the syrup. Centrifuginging then separates the sugar from the remaining liquid (molasses). Raw sugar has a yellow to brown color. To produce a white sugar, sulfur dioxide is bubbled through the cane juice before evaporation so as to bleach color-forming impurities into colourless ones. Sugar bleached white by this means is called mill white, plantation white, and crystal sugar. It is the form of sugar most often consumed in the sugarcane-producing countries.
The fibrous solids, called bagasse, remaining after the crushing of the shredded sugarcane, are burned for fuel which makes a sugar mill more than self-sufficient in energy. Any surplus bagasse can be used for animal feed, in paper manufacture, or burned to generate electricity for the local power grid.
RefiningThe second stage is the processing is done in sugar refineries, often located in heavy sugar-consuming regions such as North America, Europe, and Japan, to produce refined white sugar that is more than 99 percent pure sucrose. In such refineries, raw sugar is further purified. It is first mixed with heavy syrup and centrifuged to wash away the outer coating of the raw sugar crystals, which is less pure than the crystal interior. The remaining sugar is then dissolved to make a syrup (about 70 percent by weight solids) which is clarified by the addition of phosphoric acid and calcium hydroxide that combine to precipitate calcium phosphate. The calcium phosphate particles entrap some impurities and absorb others, and then float to the top of the tank, where they are skimmed off.
After any remaining solids are filtered out, the clarified syrup is decolorized by filtration through a bed of activated carbon. The purified syrup is then concentrated to supersaturation and repeatedly crystallized under vacuum to produce white refined sugar. As in a sugar mill, the sugar crystals are separated from the molasses by centrifuging. To produce granulated sugar, in which the individual sugar grains do not clump together, sugar must be dried. Drying is accomplished first by drying the sugar in a hot rotary dryer, and then by blowing cool air through it for several days.
The equipment used in refineries :
Refineries utilize a great many different types of physical equipment such as:
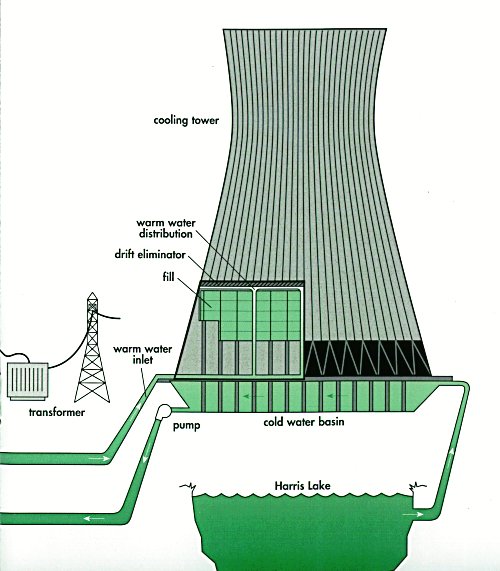
* Centrifuges * Compressors * Cooling towers * Crushers * Crystallizers * Distillation towers and other pressure vessels * Electric power generators, transformers and electric motors * Electrolysis cells * Evaporators * Filters * Furnaces * Gas flares * Mixers and blenders * Monitoring and control systems * Piping and valves * Pumps * Steam generators * Steam turbines and gas turbines * Storage tanks * Wastewater treatment